Original Article
|
|
|
|
| |
| Year : 2017 | Volume
: 5
| Issue : 3 |
Page : 28-29 |
|
|
Evaluation of film-forming potential of a Cordia dichotoma gum
V.M. Darvhekar, S.G. Jyotishi
DOI: 10.31555/jpbs/2017/5/3/28-29
Correspondence Address:Department of Pharmacognosy, P. Wadhwani College of Pharmacy, Yavatmal, Maharashtra, India, Department of Ayurvedic, Shree Ayurvedic College, Hanuman Nagar,
Nagpur, Maharashtra, India
Source of Support: None,
Conflict of Interest: Nill
 |
Check
|
DOI: 10.4103/2231-4040.197331
Films were prepared using 5 parts of 10% w/w of mucilage of gum of Cordia dichotoma with different
proportions of plasticizers: Polyethylene glycol (PEG 400) (0.1, 0.15, 0.2, and 0.3), glycerin
(0.15), and propylene glycol (0.2). The films were casted on glass plates and dried under controlled
evaporation. Films prepared with 0.15, 0.2 part of PEG 400; 0.15 part of glycerin and propylene
glycol showed satisfactory drying after 24 h. They were evaluated for following parameters water
uptake, tensile strength, folding endurance, and water vapor transmission rate. The results obtained are
comparable with films made from other polymers, and the gum can be used for preparing polymeric
drug delivery systems and as film coating agent.
Keywords: Film, gum, Cordia dichotoma
How to cite this article:
Darvhekar VM, Jyotishi SG. Evaluation of film-forming potential of a Cordia dichotoma gum. J Pharm BioSci 2017;5(3):28-29.
|
Introduction
This paper deals with the evaluation of a natural gum for its use in preparing films for application as drug delivery systems and coating agents. There are several reports about the successful use of natural gums in various pharmaceutical preparations.[1-5] The gum in the present study is an extract from the berries of Cordia dichotoma (Lasuda). The gum is initially white in color but changes to reddish brown to brownish black on exposure. It is sparingly soluble in water but swells in contact with water, giving a highly viscous solution. It is a polyuronide consisting of arabinose, galactose, and glucuronic acid in the proportion oF 10:7:L moles; rhamnose is present in traces.[6]
Materials and Methods
The gum was isolated as per the procedure reported.[7] Glycerin, propylene glycol, and polyethylene glycol (PEG 400) were obtained From S. D. Fine Chemicals, Mumbai.
Preparation of the films
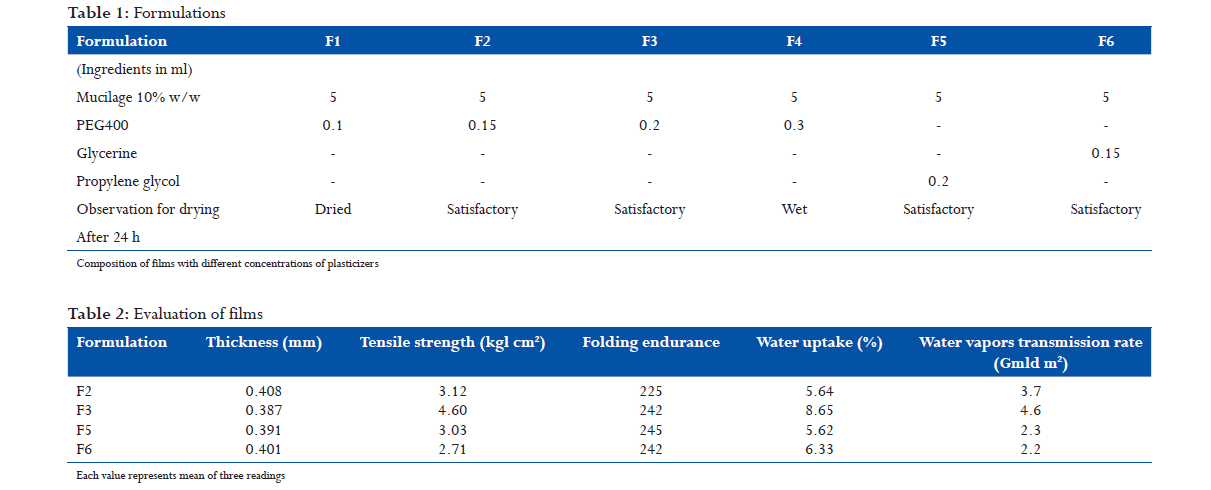
Mucilage containing 10% w/w of the gum was prepared by dispersing the gum in distilled water; it was allowed to equilibrate for 4 h. The mucilage was mixed with plasticizers, glycerin, and propylene glycol, (PEG 400) in the proportions as mentioned in Table 1, with gentle stirring for 10 min. The mixtures prepared as above were poured in glass plates, each of area 10 cm2, for casting the Films. They were allowed to dry in a closed chamber to control the evaporation for 4 h. After 4 h, the Films were observed for drying and appearance.
Evaluation of the films
The prepared films (F2, F3, F5, and F6) were evaluated for various parameters.
The water uptake was determined by drying the films at 60°C with a current of air, after which the films were subjected to desiccation over calcium chloride at 40°C for 24 h. These samples were weighed and exposed to 70% relative humidity at room temperature. This relative humidity was achieved using saturated solutions of sodium chloride. After equilibration under this humidity, films were weighed for determining the increase in weight; and percent water uptake was calculated.
The thickness of polymeric film was measured using a dial gauge (Mercer, England) having least count of 0.00L mm. The films were conditioned at 55% relative humidity at 25°C to 30°C for 48 h before testing tensile strength. To determine the elongation for calculating tensile strength, the polymeric film was pulled by means of a pulley system; weights were gradually added to the pan to increase the pulling force till the patch was broken. The elongation, i.e., the distance traveled by the pointer before breaking of the patch was noted on a graph paper with the help of magnifying glass,[8] and the tensile strength was calculated as Kg/cmL.
The folding endurance was determined using a simple instrument as reported,[9] to evaluate the ability of the films to withstand folding. The films were conditioned at 55% relative humidity at 25°C to 30°C for 48 h before testing.
Water vapor transmission rates were determined using pre-weighed glass vials of 5 mL containing 1 g of fused calcium chloride. Prepared films were fixed on the brim of the vials with an adhesive and stored in a humidity chamber at relative humidity of 70% and temperature of 25°C for 24 h; and the weight gained was determined. Water vapor transmission rate was expressed as the increase in weight of fused calcium chloride in grams (g) per day (d) of the per unit area of the film in square meters (area of the opening of the vial).
Results and Discussion
The results of evaluation study of different parameters are given in Table 2. The proportion of PEG 400 was found to have a role in the drying of the films. Films where the proportion was 0.1 mL dried up after 24 h, making the films brittle, whereas films with 0.3 mL remained wet even after 24 h of drying. Other films displayed satisfactory drying after 24 h of drying. The tensile strengths of the films were found to vary between 2.71 and 4.60 Kg/cm2. In F3, a slight increase in PEG 400 showed an increase in the tensile strength. Much variation was not observed in the folding endurance, which was found to be between 225 and 245. Water uptake was between 5.62% and 8.65%. The water uptake increased from 5.64% to 8.65% with slight increase in PEG 400. The water vapor transmission rate was found to be between 2.2 and 3.7 g/d/m2. Water vapor transmission was found to be at its maximum in F3. It can be concluded that a direct correlation exists between the PEG 400 content, moisture uptake, and water vapor transmission rate. The results obtained are comparable with the performance of polymeric Films made From hydroxypropyl methylcellulose, ethyl cellulose, Eudragit,[10] sodium carboxymethyl guar.[11]
Conclusion
From the above results, it can be concluded that gum has enormous potential for use in the preparation of polymeric films as drug delivery systems. It can also be used as a water-impervious coating agent in tablets as it has a low vapor transmission rate and satisfactory tensile strength.
References
- Tugcu-Demiröz F, Acartürk F, Takka S. Investigation of colon-specific dosage forms of ondansetron prepared with natural polymers. Pharmazie 2006;61:916-9.
- Sujja-Areevath J, Munday DL, Cox PJ, Khan KL. Release characteristics of diclofenac sodium from encapsulated natural gum matrix formulations. Int J Pharm 1996;139:53-62.
- Murali Mohan Babu GV, Prasad ChD, Ramana Murthy KV. Evaluation of modified gum karaya as carrier for the dissolution enhancement of poorly water-soluble drug nimodipine. Int J Pharm 2002;234:1-17.
- Ofoefule SI, Chukwu A. Application of Abelmoschus esculentus gum has been used as mini matrix for furosemide and diclofenac sodium tablets. Indian J Pharm Sci 2001;68:532-5.
- Lima RS, Lima JR, De Salis CR, Moreira AR. Cashew-tree (Anacardium occidentale L.) exudate gum: A novel bioligand tool. Bitechnol Appl Biochem 2002;35:45-53.
- CSIR. Wealth of India-Raw Materials. Vol. 2. New Delhi: Council of Scientific and Industrial Research; 1998. p. 429.
- Panda D, Si S, Swain S, Kanungo SK, Gupta R. Preparation and evaluation of gels from gum of Moringa oleifera. Indian J Pharm Sci 2006;68:777-80.
- Seth AK, Agarwal GP, Saini TR. Evaluation of free films. Indian Drugs 1985;23:45-6.
- Baichawal MR. Polymer films as drug delivery system. In: Proceeding of the International Symposium on Advances in Drug Delivery Systems. Mumbai; 1984. p. 128-47.
- Devi VK, Saisivam S, Maria GR, Deepti PU. Design and evaluation of matrix diffusion controlled transdermal patches of verapamil hydrochloride. Drug Dev Ind Pharm 2003;29:495-503.
- Paranjothy KL, Thampi PP. Development of ransdermal patches of verapamil hydrochloride using sodiumcarboxy methyl guar as a monolithic polymeric matrix and their in vitro release study. Indian J Pharm Sci 1997;59:49-54.
|