Original Article
|
|
|
|
| |
| Year : 2019 | Volume
: 7
| Issue : 1 |
Page : 1-7 |
|
|
Formulation and evaluation of febuxostat nanoemulsion for transdermal drug delivery
Pralhad K. Kanke, Inayat B. Pathan, Ajeet Jadhav, Md. Rageeb Md. Usman
Correspondence Address:Department of Pharmaceutics, Smt. S. S. Patil College of Pharmacy, Chopda, Maharashtra, India, Department of Pharmaceutics, Government College of Pharmacy, Aurangabad, Maharashtra, India, Department of Pharmacognosy, Smt. S. S. Patil College of Pharmacy, Chopda, Maharashtra, India
Source of Support: Nil,
Conflict of Interest: None declared
 |
Check
|
DOI: 10.4103/2231-4040.197331
The aim of the present study was to investigate the potential of nanoemulsion formulations for transdermal delivery of febuxostat. Among the various excipients tested, based on solubility study Capmul MCM, tween 80, and Transcutol P, were selected as oil, surfactant, and cosurfactant, respectively. The nanoemulsions region was identified by constructing pseudo-ternary phase diagram using aqueous phase titration. The prepared nanoemulsion was subjected to different thermodynamic stability study, and the nanoemulsion that passed thermodynamic stability tests were evaluation for viscosity, refractive index, droplet size, polydispersity index (PDI), transmission electron microscopy, and in vitro permeation study using human cadaver skin. On the basis of evaluation neutral (NE) (Z1) formulations which consist of 1.0% w/w of drug, 6.711% w/w of oil phase, 60.402% w/w of Smix, and 32.885% w/w of distilled water were selected as an optimized formulation. The mean droplet size of NE (Z1) formulation was lower (55 nm) as compared to other formulation studied. The PDI (0.33) and viscosity (30.22 ± 0.86 cp) of formulation NE (Z1) were lower as compared to other formulations. A significant increase in steady-state flux (jss) was observed in nanoemulsion NE (Z1). In conclusion, nanoemulsion could be a promising system to improve the transdermal efficacy of the febuxostat.
Keywords: Febuxostat, in vitro permeation study, nanoemulsion, transdermal drug delivery
How to cite this article:
Kanke PK, Pathan IB, Jadhav A, Usman MRM. Formulation and evaluation of febuxostat nanoemulsion for transdermal drug delivery. J Pharm BioSci 2019;7(1):1-7.
|
Introduction
Oral route still remains the favorite route of drug administration in many diseases, and till today it is the first way investigated in the development of new dosage forms. The major problem in oral drug formulations is low bioavailability, which mainly results from poor aqueous solubility. This may lead to high inter and intrasubject variability, lack of dose proportionality, and therapeutic failure.[1] The oral administration of febuxostat causes liver function abnormalities and it is contraindicated for a patient with ischemic heart disease or congestive heart failure.[2] Using the transdermal route eliminates these side effects, increases patient compliance, avoids first-pass metabolism, and maintains the plasma drug level for a longer period of time. Therefore, an improved febuxostat nanoemulsion formulation with a high degree of permeation could be useful in the treatment of locally inflamed skin and inflammatory and painful states of supporting structures of the body, such as bones, ligaments, joints, tendons, and muscles. There has been increased interest during recent years in the use of transdermal vehicles systems that could modify drug permeation through the skin.[3]
One of the most promising techniques for enhancement of transdermal permeation of drugs is the microemulsion or nanoemulsion technique.[4,5] Nanoemulsions are thermodynamically and kinetically stable, transparent (translucent) dispersions of oil and water stabilized by an interfacial film of surfactant and cosurfactant molecules having the droplet size <100 nm. Studies have shown that nanoemulsion formulations possess improved transdermal and dermal delivery properties in vitro and in vivo over emulsions and gels.
Febuxostat is chemically 2- [3- cyano-4- (2- methlypropoxy) phenyl]- 4- methlythiazole- 5 -carboxylic acid. It is a nonpurine selective inhibitor of xanthine oxidase that is indicated for use in the treatment of hyperuricemia and gout.[6]
In contrast to allopurinol, febuxostat inhibits both oxidized and reduced forms of xanthine oxidase and has minimal effects on other enzymes of purine and pyrimidine metabolism. A study comparing febuxostat to allopurinol found that more individuals treated with febuxostat had decreased levels of uric acid, but there was no difference in the amount of initial gout flares or the surface area of gout tophi.[7,8]
Materials and Methods
Febuxostat drug was a gift sample from Ajanta Pharma Ltd., Aurangabad (India). Capmul PG 8 NF (oil) was gift sample from Wockhardt Research Center, Aurangabad (India). Cosurfactant, i.e.,, Transcutol Pand Transcutol CG was gift sample from Gattefosse India Pvt., Ltd., Snatacruz East, Mumbai (India).
Some other oil such as oleic acid, Labrafac, Capryol 90, and Caster oil. Surfactant, i.e.,, Tween 80 and 20, Span 20, and Cosurfactant, i.e., ethanol, PEG 400 was gift sample from Government College of Pharmacy, Aurangabad (India).
Solubility study
The solubility of febuxostat in various oils (Capmul MCM, Labrafac, Capryol 90, and Oleic acid), surfactants (Tween 80, Tween20, and Span 80), and cosurfactant (Transcutol P, Transcutol CG, and Ethanol PEG 400) was determined by adding an excess amount of drug to 2 ml of the selected oils, surfactants, or cosurfactants separately in 5 ml stopper vials. The vials were then kept at 25 ± 1.0°C for 48 h in an isothermal shaker (Orbital Shaking Incubator, HMG, India) to reach equilibrium. The equilibrated samples were removed from the shaker and centrifuged at 3000 rpm for 5 min. The supernatant was taken and filtered through a 0.45μm membrane filter. The concentrations of febuxostat were determined in the different vehicles, i.e., oil, surfactant, and cosurfactant using ultraviolet (UV) spectrophotometer (UV-1800 double beam spectrophotometer, SIMADZU, Japan) at 315 nm. Solubility study was performed at 3 times, and the standard deviation was calculated.[1]
Pseudo-ternary phase diagram studies
On the basis of the solubility studies, the drug that has high solubility in oil, surfactant, and cosurfactant is selected for nanoemulsion. Capmul MCM was selected as the oil phase. Tween 80 and Transcutol P were selected as surfactant and cosurfactant, respectively. Distilled water was used as an aqueous phase. Surfactant and cosurfactant (Smix) were mixed in different weight ratios (1:1, 1:2, and 1:3). These Smix ratios were chosen in increasing concentration of cosurfactant with respect to surfactant for the study of the phase diagrams needed for nanoemulsion formation. For each phase diagram, oil and Smix at a specific ratio were mixed thoroughly at different mass ratios from 1:9 to 9:1 (i.e., 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1) in different glass vials. Pseudo-ternary phase diagrams of oil, Smix, and aqueous phase were developed using the aqueous titration method. Slow titration with aqueous phase was performed for each mass ratio of oil and Smix, and visual observations were made for transparent and easily Flowable o/w nanoemulsions. The physical state of the nanoemulsion was marked on a pseudo three-component phase diagram with one axis representing the oil, the second one representing a mixture of surfactant, and cosurfactant and the third representing an aqueous phase at a fixed mass ratio.[1,9,10]
Selection of nanoemulsion formulation
From each ternary phase diagram constructed, different formulation was selected from the nanoemulsion region so that the drug could be incorporated into the oil phase. Febuxostat concentration 1% (wt/wt) was kept constant in all the selected formulations.
Formulation of drug loaded nanoemulsions formulation
For the preparation of drug loaded nanoemulsions, 100 mg of febuxostat for 10 ml nanoemulsion was dissolved in the Capmul MCM. The required amount of Smix was added after that water was added dropwise until a clear and transparent liquid was obtained on ultrasonication, the composition of nanoemulsion formulations was shown in the table.
Thermodynamic stability studies of febuxostat loaded nanoemulsions formulation
To overcome the problem of metastable formulation, Thermodynamic stability tests were performed. Selected formulations were centrifuged at 3500 rpm for 30 min. Those formulations that did not show any phase separations were taken for the heating and cooling cycle. Six cycles between refrigerator temperatures of 4°C and 45°C for 48 h were done. The formulations that were stable at these temperatures were subjected to the freeze-thaw cycle test. Three freeze-thaw cycles were done for the formulations between –21°C and +25°C. Those formulations that survived thermodynamic stability tests were selected for the further studies.
Characterization of optimized nanoemulsion
The optimized nanoemulsion formulation was characterized by the various parameter.
Particle size and zeta potential measurement
The formulation (0.1 ml) was dispersed in 50 ml of water in a volumetric flask and gently mixed by inverting the flask. Globule size and zeta potential of the nanoemulsion were determined by Zetasizer HSa 3000 (Malvern instruments ltd., Malvern, U.K) that analyzes the fluctuations in light scattering due to the Brownian motion of the particles. Light scattering was monitored at 25°C at a 90° angle.[9-11]
Viscosity
The viscosity of the formulations was determined using Brookfield DV III ultra V6.0 RV cone and plate rheometer (Brookfield Engineering Laboratory, Middleboro, MA) using spindle # CPE40 at 25 ± 0.5°C.[12]
Refractive index
Refractive Index was determined using Abbe’s refractometer at 25°C.
pH
The pH of optimized febuxostat loaded nanoemulsion was determined using a digital pH meter.
Drug content
Drug content of all the formulations was >94.24 ± 0.136% and >102.54 ± 0.106%.[13]
Results and Discussion
The drug sample was characterized for its authenticity using its monograph. The drug sample was observed for nature, odor, and melting point, and solubility. The drug was identified by UV and FTIR. The drug exhibited an absorbance maximum at 315 nm which was same as the reported one. The FT-IR spectra of the febuxostat confirmed characteristic absorption bands of carboxylic acid at 2875.86/cm, hydroxyl group at 3468.01/cm, Cyanide group 2227.78/cm, and carbonyl group at 1700/cm. On the basis of these studies, it was proved that febuxostat was authentic.
Excipient selection
The excipients selected needed to be pharmaceutically acceptable, nonirritating, and nonsensitizing to the skin and to fall into the generally regarded as safe category. Higher solubility of the drug in the oil phase was another important criterion, as it would help the nanoemulsion to maintain the drug in solubilized form. Safety is a major determining factor in choosing a surfactant, as a large amount of surfactants may cause skin irritation. Nonionic surfactants are less toxic than ionic surfactants. An important criterion for selection of the surfactants is that the required hydrophilic-lipophilic balance (HLB) value to form the o/w nanoemulsion be >10. The right blend of low and high HLB surfactants leads to the formation of a stable nanoemulsion formulation. In this study, we selected Tween 80 as a surfactant with an HLB value of 15. Transient negative interfacial tension and fluid interfacial film are rarely achieved by the use of single surfactant; usually, addition of a cosurfactant is necessary. The presence of cosurfactant decreases the bending stress of interface and allows the interfacial film sufficient flexibility to take up different curvatures required to form nanoemulsions over a wide range of composition. Thus, the cosurfactant selected for the study was Transcutol P, which has an HLB value of 5(HLB-5). Febuxostat is a highly lipophilic drug, and its physicochemical properties suggest that it has good potential for transdermal drug delivery. Therefore; in the present study, different nanoemulsions were prepared for transdermal delivery of febuxostat.[1]
Solubility of febuxostat
The maximum solubility of febuxostat was found in Capmul MCM (102.14 ± 0.035 mg/ml) as compared to other oils and combinations of oils (Table 1). High drug solubility was found in Tween® 80 (247.03 ± 0.055) and Transcutol P (274.29 ± 0.065). Therefore, Tween® 80 and Transcutol P were selected as surfactant and cosurfactant, respectively, for the phase study.
Preparation of nanoemulsion formulations
For the preparation of febuxostat loaded nanoemulsions, required amount of carvedilol was dissolved in the oil phase. The required amount of mixture of surfactant and cosurfactant was added, and distilled water was then added dropwise drop till a clear and transparent liquid was obtained after ultrasonication. The prepared nanoemulsions were stored in tightly in the suitable container at ambient temperature. The selected composition of nanoemulsion formulations was shown in Table 2.
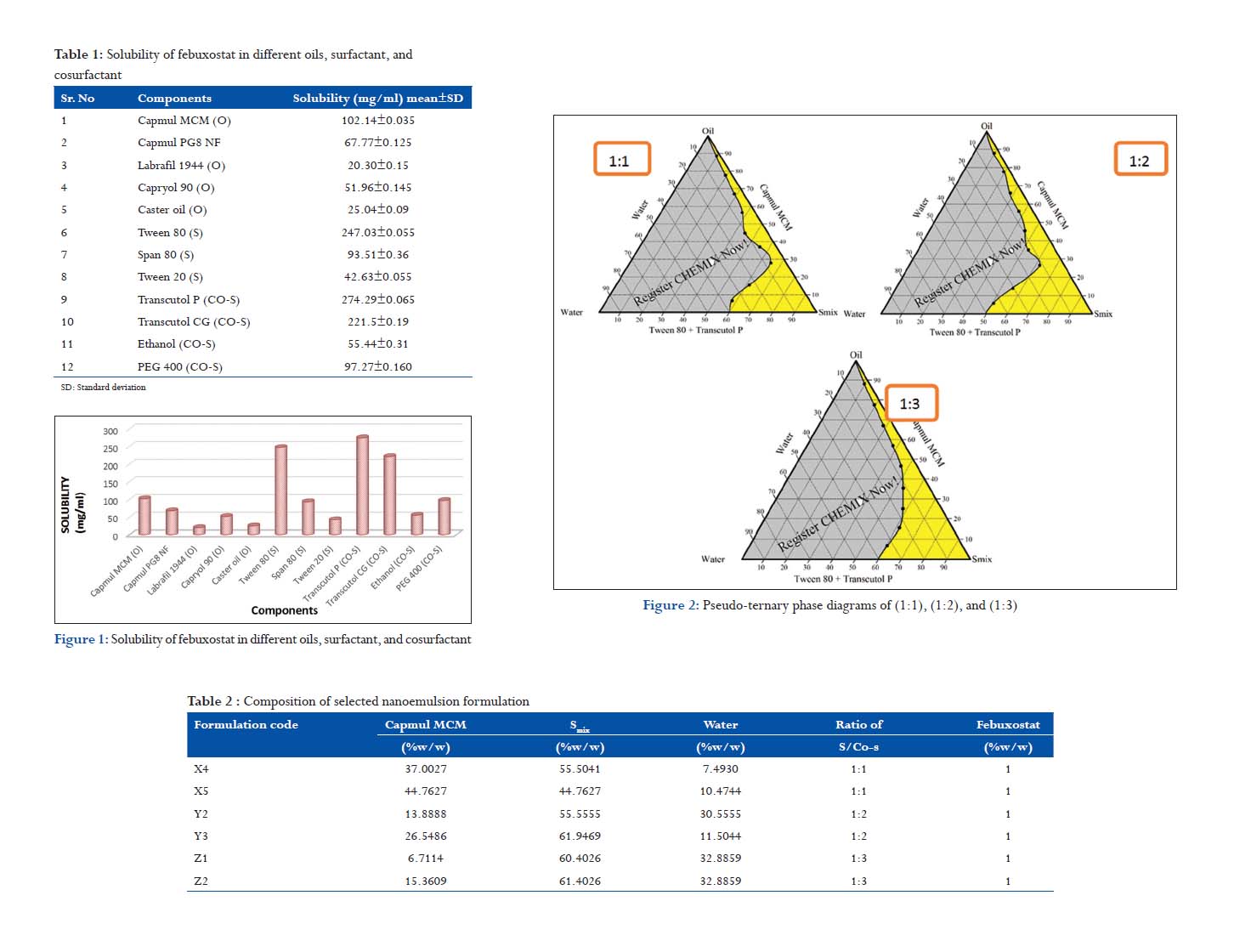
Pseudo-ternary phase diagram
A pseudo-ternary phase diagram of the investigated quaternary system Capmul MCM/Tween 80/Transcutol P/water is presented in Figure 1. Formation of nanoemulsion system (the shaded area) was observed at room temperature. Phase behavior investigation of this system demonstrated the suitable approach to determining the water phase, oil phase, surfactant concentration, and cosurfactant concentration with which the transparent, 1-phase low-viscous nanoemulsion system was formed. The phase study revealed that the less proportion of oil was incorporated in nanoemulsion systems when the surfactant to cosurfactant ratio was 1:3. Moreover, when the composition (% wt/ wt) of surfactant mixture (Smix) in a nanoemulsion preparation was <60%, the formulation was less viscous. From pseudo-ternary phase diagrams, the formulations in which the amount of oil phase completely solubilized the drug and which could accommodate the optimum quantity of Smix and distilled water were selected for the study (Figure 2).
Droplet size measurements
Droplet size was determined by photon correlation spectroscopy that analyzes the fluctuations in light scattering due to the Brownian motion of the droplets using a Delsa nano C (1000 HS, Beckmen coulter). All the nanoemulsion had small average droplet diameter between 10 and 100 nm. A small droplet sizes are very much prerequisite for drug delivery as the oil droplets tend to fuse with the skin thus providing a channel for drug delivery. Polydispersity index (PI) is a measure of particle homogeneity, and it varies from 0.0 to 1.0. The closer to zero the polydispersity value the more homogenous are the particles. Formulations showed their PI in between 0.134 and 0.394 that indicates acceptable homogeneity. Zeta potential of all nanoemulsion formulation was found between −7.02 and −0.044 mV in the 100 times diluted (Table 3). Nanoemulsion formulation consists of non-ionic components which show relatively neutral (NE) charge; it means it will not affected by body membrane charge during absorption (Figure 3).
Viscosity
The viscosity of the formulations NEB1 was determined using Brookfield DV III ultra V6.0 RV cone and plate rheometer (Brookfield Engineering Laboratory, Middleboro, MA) using spindle # CPE40 at 25 ± 0.5°C. The software used for the calculations was done by Rheocalc V2.6, and viscosity of the nanoemulsion NEB1 formulation was very low as expected for o/w emulsion (30.22 ± 0.86 cP). The low viscosity may be due to the presence of low amount of Tween-80 (a fatty acid polyhydric alcohol ester having high intrinsic viscosity) compared to Transcutol P (short chain alcohol having low intrinsic viscosity) and the low concentration of oil (Table 4).
Refractive index
Refractive Index was determined using Abbe’s refractometer at 25°C and the mean value of the refractive index for the formulation Z1 was found to be 1.31 ± 0.007.
pH
The pH of optimized carvedilol loaded nanoemulsion (Z1) was determined using a digital pH meter and was found to be 7.2 ± 0.004 which is favorable for topical application because the pH of the skin is in the range of 5.5–7.4.
Transmission electron microscopy (TEM)
The TEM studies were carried out to get more insight about the morphology of the nanoemulsion systems. The results of TEM analysis of nanoemulsion Z1 was confirmed that droplets of Z1 nanoemulsion formulation were spherical in shape and finely distributed with micron size range within 60 nm as shown in Figure 4.
In vitro skin permeation studies
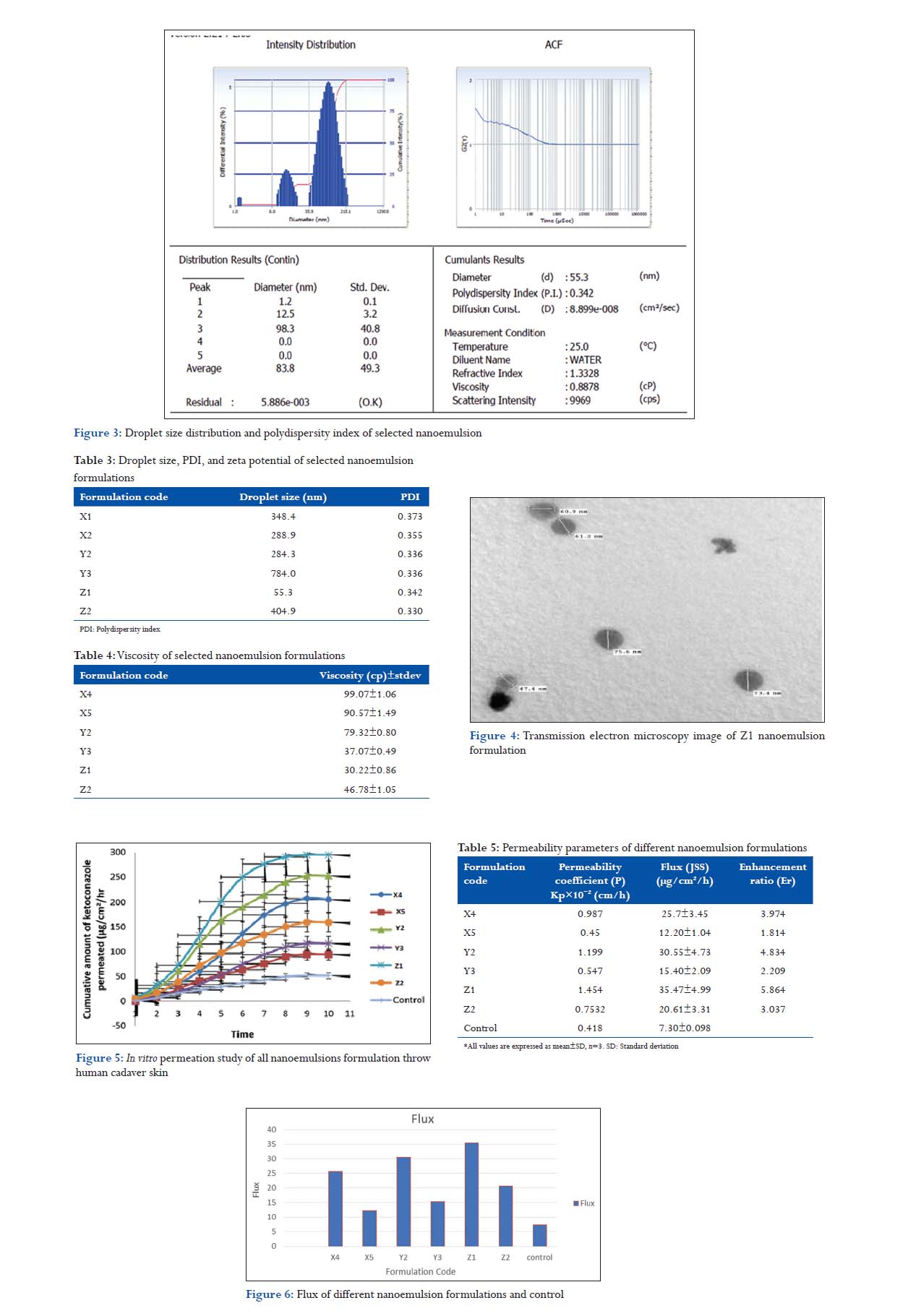
In vitro skin permeation studies were performed to compare the skin permeation of drug from six different nanoemulsion formulations (X4, X5, Y2, Y3, Z1, and Z2) and control (drug solution in phosphate buffer solution, pH 7.4), all formulations having the same quantity of Febuxostat. The permeation parameters (cumulative amount of febuxostat permeated, transdermal flux, and permeability coefficients) of selected nanoemulsion and control formulation are given in table. In vitro skin permeation was the highest in formulation Z1 and lowest X4. The nanoemulsion formulation Z1 showing highest flux (35.47 ± 4.199 μgcm−2 h−1) as shown in Table 20 and also in Figures 5 and 6. The maximum release in Z1 could be due to the smallest droplet size, i.e., (55 nm) and lowest viscosity compared to other formulation. The small droplet size and low viscosity of the nanoemulsion make it an excellent carrier for enhancing permeation uptake of febuxostat since the number of vehicle that can interact on fixed area of stratum will increase when droplet size and viscosity decrease.[12-14]
To explain the probable mechanism by which nanoemulsions enhance the skin permeation of drugs, the histological and histochemical structure of stratum corneum must be taken into consideration. Drugs permeate stratum corneum through two micro pathways, i.e.,, intercellular and transcellular pathways. Of these, the intercellular pathway plays a major role in percutaneous uptake of drugs. It is well known that a complex mixture of essentially NE lipids, which are arranged as a bilayer with their hydrophobic chains facing each other, forms a lipophilic bimolecular leaflet. Most of the lipophilic drugs pass through this region, and it is called a lipid pathway. The polar head group of lipids faces an aqueous region, forming a polar route that hydrophilic drugs generally prefer. A dermally applied an emulsion is expected to penetrate the stratum corneum and to exist intact in the whole horny layer, alter both lipid and polar pathways (26). The drug dissolved in the lipid domain of the nanoemulsions can directly penetrate the lipid of the stratum corneum, thereby destabilizing its bilayer structure. These interactions will increase the lipid pathway permeability to drugs. On the other hand, the hydrophilic domain of nanoemulsions can hydrate the stratum corneum to a greater extent and play an important role in the percutaneous uptake of drugs. When the aqueous fluid of nanoemulsions enters the polar pathway, it increases the interlamellar volume of the stratum corneum lipid bilayer, resulting in disruption of its interfacial structure. A lipophilic drug like FEBUXOSTAT can then permeate more easily through the lipid pathway of stratum corneum. Moreover, droplet size and viscosity of the nanoemulsion may also affect its efficiency, where the small droplet size and low viscosity of the nanoemulsion make it an excellent carrier for enhancing percutaneous uptake of FEBUXOSTAT, since the number of vesicles that can interact on a fixed area of stratum corneum will increase when droplet size and viscosity decrease. Therefore, the probable reason for enhanced permeation of FEBUXOSTAT from T1 to T2 could be the combined effects of hydrophilic and lipophilic domains as well as the smallest droplet size and lowest viscosity of nanoemulsions. Permeability parameters of different nanoemulsion formulations are shown in Table 5.
Conclusion
We have successfully prepared and characterized febuxostat nanoemulsion for transdermal permeation. On the basis of highest drug release, lowest droplet size, thermodynamic stability and optimum surfactant, and cosurfactant concentration. On the basis of the in vitro drug release studies the formulation Z1, which contained Capmul MCM 6.711%, Tween 80, and Transcutol P (Smix) 60.40% and distilled water 32.88% was optimized. From the in vitro data, it can be concluded that the developed nanoemulsions have great potential for transdermal drug delivery.
Acknowledgment
The authors express gratitude to Ajanta Pharma Ltd., for providing gift sample of febuxostat Aurangabad (India), Wockhardt Research Center, Aurangabad (India), for providing gift sample of Capmul PG 8 NF (oil) and Government College of Pharmacy, Aurangabad, for providing Zeta sizer for Partical size measurement.
References
- Patel PK, Patel MR, Patel KR. Design and development of self-microemulsifying drug delivery system of febuxostat. Int J Univ Pharm Bio Sci 2014;3:285-99.
- Modi J, Jayvadan P. Nanoemulsion-based gel formulation of aceclofenac for topical delivery. Int J Pharm Pharm Sci Res 2011;1:6-12.
- Guy RH. Current status and future prospects of transdermal drug delivery. Pharm Res 1996;13:1765-9.
- Jain NK. Controlled and Novel Drug Delivery System. 1st ed. New Delhi: CBS Publications; 1997. p. 110-5.
- Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M, et al. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm 2007;66:227-43.
- Izquierdo P. Formation and stability of nanoemulsions. Adv Colloids Interface Sci 2004;108-109:303-18.
- Haritha M, Basha SP, Rao PK, Vedantham C. A brief introduction to methods of preparation, application and characterization of nanoemulsion drug delivery systems. Indian J Res Pharm Biotechnol 2013;1:25-8.
- Verma S. Formulation and characterization of Febuxostat loaded microemulsion for the treatment of rheumatoid arthritis. World J Pharm Res 2014;3:4305-35.
- Kavita S, Singh SK. Formulation development and optimization of selfemulsifying drug delivery system (SMEDDS) of Meloxicam. Int J Pharm Pharm Sci 2013;5:524-30.
- Reza KH. Khussanreza, nanoemulsion as a novel transdermal drug delivery system. Int J Pharm Sci Res 2011;2:1938-46.
- Kriwet K, Müller-Goymann CC. Diclofenac release from phospholipid drug systems and permeation through excised human stratum corneum. Int J Pharm 1995;125:231-42.
- Tanojo H, Junginger HE, Boddé HE. In-vivo human skin permeability enhancement by oleic acid: Transepidermal water loss and Fourier-transform infrared spectroscopy studies. J Control Release 1997;47:31-9.
- Shakeel F, Baboota S, Ahuja A, Ali J, Shafiq S. Skin permeation mechanism and bioavailability enhancement of celecoxib from transdermally applied nanoemulsion. J Biotech 2008;16:733-40.
- Pershing LK, Parry GE, Lambert LD. Disparity of in vitro and in vivo oleic acidenhanced b-estradiol percutaneous absorption across human skin. Pharm Res 1993;10:1745-50.
|