Original Article
|
|
|
|
| |
| Year : 2018 | Volume
: 6
| Issue : 4 |
Page : 50-54 |
|
|
Synergistic effect of herbal plants in diabetic rats from Satpuda region
Kiran D. Patil, V. Vaidhyalingam, K. L. Shentilkumar
Correspondence Address:Department of Pharmacology, Tamil Nadu Dr. M. G. R. Medical University, Chennai, Tamil Nadu, India
Source of Support: Nil,
Conflict of Interest: None declared
 |
Check
|
DOI: 10.4103/2231-4040.197331
The incidence of diabetes mellitus is reportedly on the rise, especially in the developing countries, and it is estimated that these countries will witness a 69% increase between 2010 and 2030. A high cost of medical care of diabetes is forcing an increasing number of people into the use of herbal alternatives for cure. Till now, so many researchers have evaluated many plants for their antihyperglycemic and antihyperlipidemic activities. However, still, we are lacking to prepare effective ayurvedic dosage form which can complete the allopathic drugs. This difficulty can be overcome using the synergism. Synergism can be defined as the interaction or cooperation of two or more substances to produce a combined effect greater than of their separate effects. Hence, in this present study, we therefore assessed the combinatorial effect of the extracts of fresh fruit of Lagenaria siceraria and Eulophia herbacea in diabetic rats. The significant reduction of glucose and lipid levels in combinatorial extracts was superior as compared with the respective monotherapies. Finally, the combinatorial effect of extracts proved the hypothesis of the synergistic effect of selected plants.
Keywords: Hyperglycemia, Lagenaria siceraria, Eulophia herbacea, cholesterol, alloxan Introduction
How to cite this article:
Patil KD, Vaidhyalingam V, Shentilkumar KL. Synergistic effect of herbal plants in diabetic rats from Satpuda region. J Pharm BioSci 2018;6(4):50-54
|
Introduction
Diabetes mellitus is a group of metabolic diseases characterized by high blood sugar (glucose) levels that result from defects in insulin. Normally, blood glucose levels are tightly controlled by insulin, a hormones produced by the pancreas. Insulin lowers the blood glucose level. When the blood glucose elevates (e.g., after eating food), insulin is released from the pancreas to normalize the glucose level. In patients with diabetes, the absence or insufficient production of insulin causes hyperglycemia.[1] Diabetes is a chronic medical condition, called as silent killer because it is often diagnosed too late on the damage may already have been done. It impacts not only people with the disease but also their families and costing societies heavily in treating many serious complication that arise in undiagnosed or poorly rated diabetes.[2]
The incidence of diabetes mellitus is reportedly on the rise, especially in the developing countries, and it is estimated that these countries will witness a 69% increase between 2010 and 2030.[3] According to the International Diabetes Federation (IDF), diabetes is turning out to be bigger monster than aids. As for the recent statistics released by IDF, every ten seconds, a person dies from diabetes-related causes across the world. Every year 3.8 million people die of this disease, and more than 246 million people ranging from 20 to 79 years live with diabetes.[4] the World Health Organization (WHO) estimates that by 2025 as many as 200–300 million people worldwide will develop diabetes.[5] Pathogenesis of diabetes mellitus is managed by insulin and oral administration of hypoglycemic drugs such as sulfonylureas and biguanides.[2] Development of an adverse event is one of the complications in the treatment of any systemic disorder; hence, many of the research institutes and pharmaceutical companies are involved in drug development to find the molecules with good therapeutic potential and less adverse events.[6] Toxicity of oral antidiabetic agents differs widely in clinical manifestations, severity, and treatment. The use of herbal medicines for the treatment of diabetes mellitus has gained importance throughout the world. The WHO also recommended and encouraged this practice, especially in countries, where access to the conventional treatment of diabetes is not adequate. There is an increased demand to use natural products with antidiabetic activity due to the side effects associated with the use of insulin and oral hypoglycemic agents. The available literature showed that there are more than 400 plant species having hypoglycemic activity.[7] Therefore, it is a need of the day to search other materials from natural sources that are less toxic and less expensive and provides better safety and efficacy on long use. Herbal medicines have been used for many years by different cultures around the world, for both the prevention and management of diabetes.[8,9] The concept of polyherbal is well documented in the ancient literature. Compared to the single herb, the combination has better and extended therapeutic potential.[10] It is believed that the synergistic interactions between the constituents are responsible for the therapeutic efficacy.[11-13] Hence, the present study was planned to study the synergistic effect of a mixture of methanolic extract of plants having known antidiabetic activity and evaluate its therapeutic effects in rodents.
Materials and Methods
Plant collection and authentication
The plant material Eulophia herbacea Lindl., family – Orchidaceous, was collected from Toranmal region, Nandurbar District, Maharashtra, India, in July–August.
The second plant material Lagenaria siceraria was collected from local farm of Satpuda region, Dhule district, Maharashtra.
The plants specimen of E. herbacea Lindl. and L. siceraria Mol. was authenticated by Dr. D. A. Patil Sir, Department of Botany, S S V P S College of Arts, Commerce, and Science, Dhule, Maharashtra.
Extraction using Soxhlet apparatus
Tubers of E. herbacea and fresh fruit of L. siceraria were crushed separately. The crushed materials of both plants were defatted with petroleum ether (60–800). Approximately 500 g of the defatted material of each was extracted with methanol in several batches using Soxhlet apparatus. After extraction by methanol, the crushed material was removed from the thimble and dried. Final extracts were filtered and concentrated under reduced pressure in a rotary flash vacuum evaporator to get crude methanolic extract (CME) of both plants. The dried extracts were then collected and preserved in desiccators.[14,15]
Experimental animals
Rats were chosen as it accumulates cholesterol in both the liver and bloodstream somewhat more than mouse.[16] Lipids play an important role in the pathogenesis of diabetes mellitus.[17] Most of the studies were reported on Wistar rats. Hence, adult Wistar rat (150–200 g) of either sex was selected as experimental animal. The animals were housed under standard conditions of temperature (22 ± 1°C), relative humidity (55 ± 10%), and 12 h light/dark cycles and fed with standard pellet diet and water ad libitum. The study protocol was approved by the Institute Animal Ethics Committee of the Padmavathi College of Pharmacy, Dharmapuri 1143/ac/07/CPCSEA/IACE/ Ph.D/118/12.di
Acute toxicity studies
The procedure was followed using the Organization of Economic Cooperation and Development guidelines. Overnight fasted rats were used for the study. Rats were divided into three groups. CME of LS and EH individually tested for the toxicity using defined doses in increasing order (5, 50, 300, and 2000 mg/kg body weight) and combinatorial extract in 1:1 ratio for each defined doses were tested separately.[18]
Optimization of diabetes mellitus in rats
Diabetes was induced in overnight fasted experimental animals (rats of either sex) by intraperitoneal injection of alloxan monohydrate (150 mg/kg). After 48 h of alloxan injection, the blood sample is withdrawn by retro-orbital method and blood glucose level is determined to confirm diabetic. The diabetic-induced rats only selected for the further study.[19]
Experimental design
The above optimized diabetic rats were selected for the actual study. Selected rats were divided into five groups. Each group consists of six animals. The grouping of the experimental diabetic rats was done as follows:
- Group I: Alloxan monohydrate (150 mg/kg)
- Group II: Alloxan monohydrate (150 mg/kg) + methanolic extract of LS (300 mg/kg)
- Group III: Alloxan monohydrate (150 mg/kg) + methanolic extract of EH (300 mg/kg)
- Group IV: Alloxan monohydrate (150 mg/kg) + combinatorial methanolic extract of LS and EH (mixture of 150 mg/kg of EH and 150 mg/kg LS)
- Group V: Alloxan monohydrate (150 mg/kg) + metformin (150 mg/kg) n = 6 rats each.
The standard drug metformin and the extracts were suspended in 1% w/v carboxymethylcellulose and administered once daily through oral gavage for 21 consecutive days. The blood samples were collected on 1st, 7th, 14th, and 21st days of the treatment, through the tail vein of rats by pricking, and were immediately used for the estimation of blood glucose with a glucometer. Weekly body weight variations were monitored for all the experimental animals.
At the end of the experiment, the blood sample was withdrawn from all the experimental animals through retro-orbital plexus puncture/ posterior vena cava in plain and sodium ethylenediaminetetraacetic acid tubes for biochemical chemical estimation of glucose, cholesterol, and triglycerides.[20]
Statistical analysis of data
Data were expressed as mean ± standard error of the mean. Statistical analysis was carried out with one-way ANOVA followed by Dunnett’s multiple comparison test using GraphPad prism software. A level of significance of P < 0.05 was considered as statistically significant.
RESULTS
Toxicity study of CME of plants
Acute toxicity studies did not show any mortality or symptoms of toxicity up to 2000 mg/kg given as single oral administration. Hence, the study was carried out at the dose levels of 300 mg/kg individually and 150 mg/kg in extract combination.
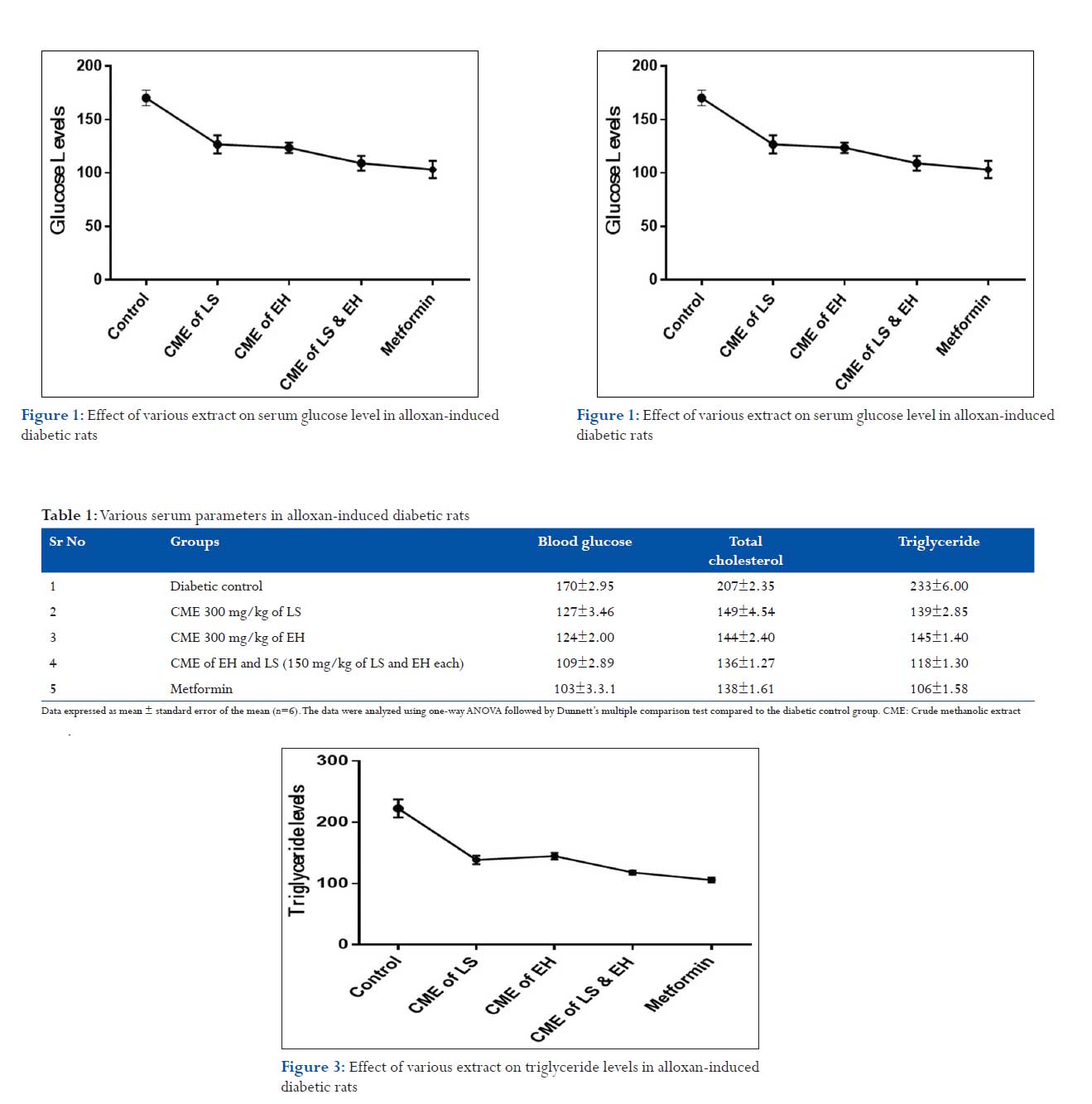
The data presented in Table 1 represent the blood glucose levels, total cholesterol level, and triglyceride levels of diabetic control rats, extract-treated diabetic rats, and standard drug (metformin)-treated diabetic rats. The levels of diabetic control rats showed hyperglycemia in comparison to normal glucose levels. Figure 1 represents that mean blood glucose level in the diabetic control group on 21st day was 170 ± 2.95 mg/dl. The mean blood glucose level in the extract treated groups, i.e.,, CME of L. siceraria (300 mg/kg) and CME of E. herbacea (300 mg/kg) observed on the 21st day was 127 ± 3.46 and 124 ± 2.00, respectively. Furthermore, it was observed that the standard drug metformin lowered the blood glucose level significantly, bringing it below the normal level up to 103 ± 3.31, whereas the combinatorial of CME of L. siceraria and E. herbacea-treated group significantly (P < 0.0001) decreased the fasting blood serum glucose level in the diabetic rats on 21st days to 109 ± 2.89, as compared to the diabetic control group.
Effects of CME extract on lipid profile in diabetic rats
The induction of diabetes caused a significant increase in triglycerides, total cholesterol, and low-density lipoprotein-cholesterol concentrations (Table 1). Figures 2 and 3 represent that the administration of CME of LS and CME of EH at dose of 300 mg/kg individually in Groups II and III significantly reversed the situation by decreasing the serum total cholesterol (P < 0.0001) to 149 ± 4.54 and 144 ± 2.40, respectively, in comparison to diabetic control group, i.e., 207 ± 2.35, whereas the triglyceride level to 139 ± 2.85 and 145 ± 1.40, respectively, comparison to diabetic control group, i.e., 233 ± 6.00. Similarly, more significant reduction in the total cholesterol level in combinatorial extract-treated group and standard control group of metformin was noticed. Combinatorial extracttreated group shows better reduction in the total cholesterol level and triglyceride level to 136 ± 1.27 and 118 ± 1.30, respectively, than that of individually extract-treated groups. Finally, it is confirmed that methanolic extract of LS and EH is effective in the prevention of diabetic mellitus and hyperlipidemia. Hence, the synergistic effect at the lesser concentration of the methanolic extract of both plants in 1:1 ratio was notified.
Discussion
The combinatorial mixture was prepared using the methanolic extract of the fresh fruit of L. siceraria and the tubers of E. herbacea in combination of 1:1 ratio. The antidiabetic activity of individual plants has been proven. The methanolic and aqueous extracts of E. herbacea at the dose of 200 mg/kg and 400 mg/kg in alloxan-induced diabetic rats were reported.[21] Antidiabetic activity of L. siceraria pulp extract and seed extract was evaluated in alloxan-induced diabetic rats.[22]
In the modern era, herbal formulations have gained greater importance than ever before, mainly due to their efficacy and easy availability as well as less side effects as compared to the synthetic drugs.[23] These advantages have led the people to move toward herbal preparations, for disease treatment and prevention, as they are claimed to display synergistic, potentiative, and agonistic/antagonistic actions, and the mixture of species in them shows better therapeutic effect than either species on its own.[24] The concept of polyherbalism has been highlighted in Sharangdhar samhita, an Ayurvedic literature dating back to 1300 AD.[25] Polyherbal formulations enhance the therapeutic action and reduce the concentrations of single herbs, thereby reducing the adverse events.
Numerous studies have found that the blood glucose level increased significantly in animals that were injected with alloxan.[26,27] Hyperglycemia was induced in experimental overnight fasted rats by single intraperitoneal injection of freshly prepared alloxan monohydrates in normal saline (150 mg/kg) body weight. The serum glucose levels were estimated to confirm the hyperglycemia after 48 h injection.[19]
It is well established that alloxan administration to experimental rats selectively causes pancreatic cell membrane disruption and ultimately cytotoxicity after its intracellular accumulation.[28] Alloxan causes a massive destruction of β-cells of the islets of Langerhans, resulting in reduced synthesis and release of insulin.[29] Hyperglycemia is a marker symptom of diabetes. It is well known that, during the evolution of diabetes mellitus in rats, after administration of alloxan, the function of the insulin system is suppressed, which is expressed by a high level of hyperglycemia.[30] Expression of elevated blood fasting glucose levels confirmed induction of diabetes in alloxoninduced experimental rats.
Diabetes is associated with hyperlipidemia.[31] In diabetes, more free fatty acids release into the circulation. Increased fatty acid concentration also increases the β-oxidation of fatty acids, producing more acetyl-CoA, and cholesterol.[17] It is well known that insulin activates enzyme lipoprotein lipase, which hydrolyzes triglyceride under normal conditions. Destruction of betacells leads to the depletion of plasma insulin, which results in hyperlipidemia.[32]
Hence, in the present study, the lipid parameter along with glucose levels was estimated in the diabetic rats. The significant control of plasma lipid levels suggests that the extract may produce its action by improving insulin secretion.
In the diabetic control group, we found consistent hyperglycemia (blood sugar level >150 mg/dl) from the 5th day post-alloxan injection up to the end of the study. Extract-treated groups were compared with diabetic control group. The significant reduction in blood glucose levels in extract-treated groups was initially estimated. However, more significant result of combinatorial extract is reported.
Conclusion
Thus, our study findings demonstrate the synergistic effect in alloxan‑induced diabetic rats using combinatorial methanolic extract of L. siceraria and E. herbacea in 1:1 ratio.
Hence, the effective action of plants in combination is beneficial in managing diabetes and hyperlipidemia.
References
- Tripathi KD. Essentials of Medical Pharmacology. 7th ed. New Dehli: Jaypee Brothers Medical Publisher; 2013. p. 258-62.
- Gilman AG, Goodman LS. The Pharmacological Basis of Therapeutics. 10th ed. New York: Macmillan; 1985. p. 1689-710.
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4-14.
- IDF. International Diabetes Federation. IDF Diabetes. 7th ed. Brussels, Belgium: International Diabetes Federation; 2015.
- Bangar AV, Saralaya MG. Anti-hyperglycaemic activity of ethanol extract and chloroform extract of Indigofera tinctoria leaves in streptozotocin induced diabetic mice. Res J Pharm Biol Chem Sci 2011;2:445-55.
- Parasuraman S, Kumar E, Kumar A, Emerson S. Free radical scavenging property and diuretic effect of triglize, a polyherbal formulation in experimental models. J Pharmacol Pharmacother 2010;1:38-41.
- Islam MA, Akhtar AM, Khan MR, Hossain MS, Alam MK, Wahed MI, et al. Antidiabetic and hypolipidemic effects of different fractions of Catharanthus roseus (Linn.) on normal and streptozotocin-induced diabetic rats. J Sci Res 2009;1:334-44.
- Jung M, Park M, Lee HC, Kang YH, Kang ES, Kim SK. Antidiabetic agents from medicinal plants. Curr Med Chem 2006;13:1203-18.
- Kim JD, Kang SM, Park MY, Jung TY, Choi HY, Ku SW. Ameliorative antidiabetic activity of dangnyosoko, a Chinese herbal medicine in diabetic rats. Biosci Biotechnol Biochem 2007;71:1527-34.
- Ulrich-Merzenich G, Panek D, Zeitler H, Vetter H, Wagner H. Drug development from natural products: Exploiting synergistic effects. Indian J Exp Biol 2010;48:208-19.
- Scholey AB, Kennedy DO. Acute, dose-dependent cognitive effects of ginkgo biloba, panax ginseng and their combination in healthy young volunteers: Differential interactions with cognitive demand. Hum Psychopharmacol 2002;17:35-44.
- Petchi RR, Vijaya C, Parasuraman S. Antidiabetic activity of polyherbal formulation in streptozotocin - nicotinamide induced diabetic wistar rats. J Tradit Complement Med 2014;4:108-17.
- Williamson EM. Synergy and other interactions in phytomedicines. Phytomedicine 2001;8:401-9.
- Dewanjee S, Bose S, Sahu R, Mandal SC. Antidiabetic effect of matured fruits of Diospyros perigrina in alloxan induced diabetic rat. Int J Green Pharm 2008;2:95-9.
- Mukherjee PK. Quality Control of Herbal Drugs. New Delhi: Business Horizons; 2002. p. 67-92.
- Beher WT, Baker GD, Penny DG. A comparative study of the effects of bile acids and cholesterol on cholesterol metabolism in the mouse, rats, hamster and Guienia pig. J Nutr 1963;73:523-30.
- Ozturk Y, Altan V. Diabetic complications in experimental models. Tr J Med Sci 1998;22:331-41.
- Anonymous. OECD Guidelines for Testing of Chemicals: Acute Oral Toxicity – Acute Toxic Class Method. Paris, France: OECD. 2001. p. 1-14.
- Mohammed FA, Kazim SM, Ghori SS, Mehjabeen SS, Ahmed SR, Ali SM, et al. Antidiabetic activity of Vinca rosea extracts in alloxan-induced diabetic rats. Int J Endocrinol 2010;2010:1-6.
- Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother 2010;1:87-93.
- Tatiya AU, Puranik PM, Surana SJ, Patil YS, Mutha RE. Evaluation of hypolipidemic, Antiglycemic and antioxidant activity of Eulophia tubers. Bangladesh J Pharmacol 2013;8:229-75.
- Bhattacharya S, Das B. Anti-diabetic activity of Lagenaria siceraria pulp and seed extract in normal and alloxan-induced diabetic rat. Int J Pharm Sci Res 2012;3:3362-9.
- Katare YS, Bhujbal SS, Bafna AR, Shyale SS, Shelar MK, Kadam SD, et al. Evaluation of anxiolytic effect of a polyherbal for mulation in mice. Eur J Exp Biol 2012;2:2093-8.
- Sujatha S, Shalin JJ. Complementary therapeutic potential: A focus on polyherbal products for hyperglycemia. Asian J Sci Res 2012;5:1-13.
- Srivastava S, Lal VK, Pant KK. Polyherbal formulations based on Indian medicinal plants as antidiabetic phytotherapeutics. Phytopharmacology 2012;2:1-15.
- Shivarajan VV, Balachandra I. Ayurvedic Drugs and their Plant Source. New Delhi: Oxford and IBH Publishers; 1996. p. 176-7.
- Lenzen S, Panten U. Alloxan: History and mechanisms of action. Deabetologica, 1998;31:337-42.
- Lu H, Chen J, Li W, Ren B, Wu J, Kang H, et al. Hypoglycemic and hypolipidemic effects of the total triterpene acid fraction from Folium eriobotryae. J Ethnopharmacol 2009;122:486-91.
- Iams SG, Wexler BC. Alloxan diabetes in spontaneously hypertensive rats: Gravimetric, metabolic and histopathological alterations. Br J Exp Pathol 1977;58:177-99.
- Lenzen S. The mechanisms of alloxan and streptozotocin induced diabetes. Diabetologica 51, (2008), 216-226.
- Chase PH, Glasgow AM. Juvenile diabetes mellitus and serum lipid and lipoprotein levels. Am J Dis Child 1976;130:1113-7.
- Aruna P, Roopa K.Evaluation of antidiabetic activity of Cassia auriculata Linn seeds for alloxan induced diabetes in rats. J Pharm Res Opin 2011; 1;30-3.
|